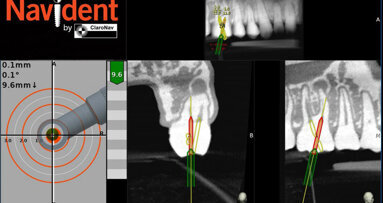
TORONTO, Ontario, Canada: ClaroNav Inc. recently announced that it has received 510(K) clearance from the Food and Drug Administration (FDA) to market and sell Navident, its navigation system for dental implantations, in the United States. Using the CBCT image as a map, Navident guides surgeons just like a GPS guides drivers. The dental surgeon plans where implants should be placed in the image and Navident, dynamically tracks the drill and the patient’s jaw providing guidance and feedback.
Headquartered in Toronto, ClaroNav has been successfully developing and marketing imaging and navigation components and clinical solutions to the global medical community for over 15 years. Navident is the result of close collaboration between ClaroNav and the University of Toronto’s Faculty of Dentistry.
“While surgical navigation has long been the accepted standard of care in neurosurgery and sinus surgery, its use in the dental field has thus far been limited. Leveraging our proven tracking and image processing components and years of clinical research, we have created a highly accurate, portable and affordable system that we are confident will make the process of restoring missing teeth more predictable, affordable and less painful for American patients.” said Doron Dekel, CEO of ClaroNav Inc.
Navident has been successfully marketed and sold in Europe, Asia and Canada for the past 18 months with great success. A clinical usage study, submitted as part of Navident’s FDA application, asked surgeons from these various regions to provide case reports, accuracy assessments and general feedback on the system. Average reported guided placement accuracy of about 1.0mm at the entry point and apex of the implant, and the highly positive survey results provided strong evidence for the following claims:
- Navident is safe: Usage errors that may lead to incorrect guidance are rare and are caught by the accuracy check prior to drilling.
- Navident is effective: It boosts surgeon’s confidence in the quality and safety of the implantations and enables flapless surgery.
- Navident is easy to use: Users are satisfied or very satisfied with all key aspects of Navident’s design.
To provide effective training of American dentists in the optimal use of Navident, ClaroNav plans to extend its global Dynamic Navigation Society to the US, where it will establish a network of Master Clinical Trainers and conduct regular training courses in all regions of North America.
Jason Pardo, ClaroNav’s vice president of sales and marketing, added: “Dental treatment in general, and dental implant treatment in particular, changes people’s lives for the better. We are proud to be able to provide a solution that contributes to the accuracy, safety and comfort of the implant treatment and are excited about making it available to the large and sophisticated US dental market. Many American dental surgeons are global leaders in the adoption of new technologies and techniques, so obtaining the privilege of serving their needs has positive implications for the future of Navident in the global market as well.”
About ClaroNav Inc.
ClaroNav (formerly Claron Technology Inc.), founded in 2001, focuses on surgical navigation, offering instrument positioning guidance solutions to clinicians in the treatment of their patients. ClaroNav’s Navident system is setting the standard for surgical navigation technology in the dental industry to make implant treatment safe and secure. The privately owned, self-funded, company, is represented worldwide by subsidiary offices and authorized distributors and is a trusted partner for many clinics and research institutions.
TORONTO, Ontario, CANADA: ClaroNav announced today (June 3, 2020) that it has obtained Health Canada and CE Mark approvals to expand the indications for use...
GLASGOW, Scotland: New biocompatible materials for 3D printing and milling that employ the unique Remora anti-biofilm technology have gained clearance from ...
OCO Biomedical, based in Albuquerque, N.M., a manufacturer of dental implants and attachment systems, recently received approval from Health Canada for the ...
Dr. Mario Brondani, a dentistry professor at the University of British Columbia (UBC), has received a grant of $67,000 from Genome BC to create structural ...
TORONTO, Ontario, Canada: Less than one per cent of healthy urban children surveyed in Toronto had received dental care by the recommended age of 12 months ...
Visitors can explore a full portfolio of new and cutting-edge products, engage in hands-on demos with a knowledgeable team of product experts and take ...
The last time the Ontario Dental Association held its Annual Spring Meeting, back in 2019, the world was a different place. So it’s fitting that this ...
In 2011, the Canadian Dental Association and the provincial dental associations launched a joint national advertising campaign reinforcing the value of ...
TORONTO, Ontario, CANADA: For years, the Ontario Dental Association has made it clear that it wants to work with the province to make sure Ontarians get the...
OTTAWA, Ontario, CANADA: In a public statement issued today (Sept. 21, 2021), the Canadian Dental Association (CDA), the national voice for dentists across ...
Prof. Dr. Marcel A. Wainwright DDS, PhD
Live webinar
Wed. 25 February 2026
11:00 AM EST (Toronto)
Prof. Dr. Daniel Edelhoff
Live webinar
Wed. 25 February 2026
1:00 PM EST (Toronto)
Live webinar
Wed. 25 February 2026
8:00 PM EST (Toronto)
Live webinar
Tue. 3 March 2026
11:00 AM EST (Toronto)
Dr. Omar Lugo Cirujano Maxilofacial
Live webinar
Tue. 3 March 2026
8:00 PM EST (Toronto)
Dr. Vasiliki Maseli DDS, MS, EdM
Live webinar
Wed. 4 March 2026
12:00 PM EST (Toronto)
Munther Sulieman LDS RCS (Eng) BDS (Lond) MSc PhD



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 International / International
International / International
 Brazil / Brasil
Brazil / Brasil
 Latin America / Latinoamérica
Latin America / Latinoamérica
 USA / USA
USA / USA
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East















































To post a reply please login or register